At Vircell, we offer under our registered trademark AMPLIRUN® third opinion molecular controls for molecular diagnostic testing of infectious diseases
Two kinds of molecular controls. One Mission
AMPLIRUN®
Purified inactivated whole genome
Validation of NAAT amplification and detection steps.
Content:
The kit includes 2 vials*:
- 1 vial containing lyophilized DNA/RNA of the infectious agent.
- 1 vial of DNAse/RNAse-free certified reconstitution solution to resuspend the control before use.
Quantification:
Precise information about the control concentration is included in the product documentation:
- DNA quantified references: 10,000 – 20,000 copies/µL
- RNA quantified references: 12,500 – 20,000 copies/µL
Shelf-life:
2.5 years for DNA and 2 years for RNA, when stored as indicated.
(* A third vial with specific primers will be included in the DNA control reference based on a plasmid)
AMPLIRUN®TOTAL
Complete, inactivated microorganism in a matrix that mimics the human sample
Validation of complete workflow – sample preparation (extraction), amplification and detection. Routine use recommended.
Content:
- 10 single-use lyophilized vials.
- These controls mainly consist of panels, adapted to relevant market tests.
Quantification:
- Low positive controls (20-50 c/µl)
- Formulated to produce results at a clinical significant concentration
- Great lot-to-lot consistency
Shelf-life:
Up to 2.5 years from date of manufacture. Once reconstituted the control should be refrigerated and used within 12 hours.
Matrix:
Plasma, swab, sputum, exudate, urine, stool and CSF
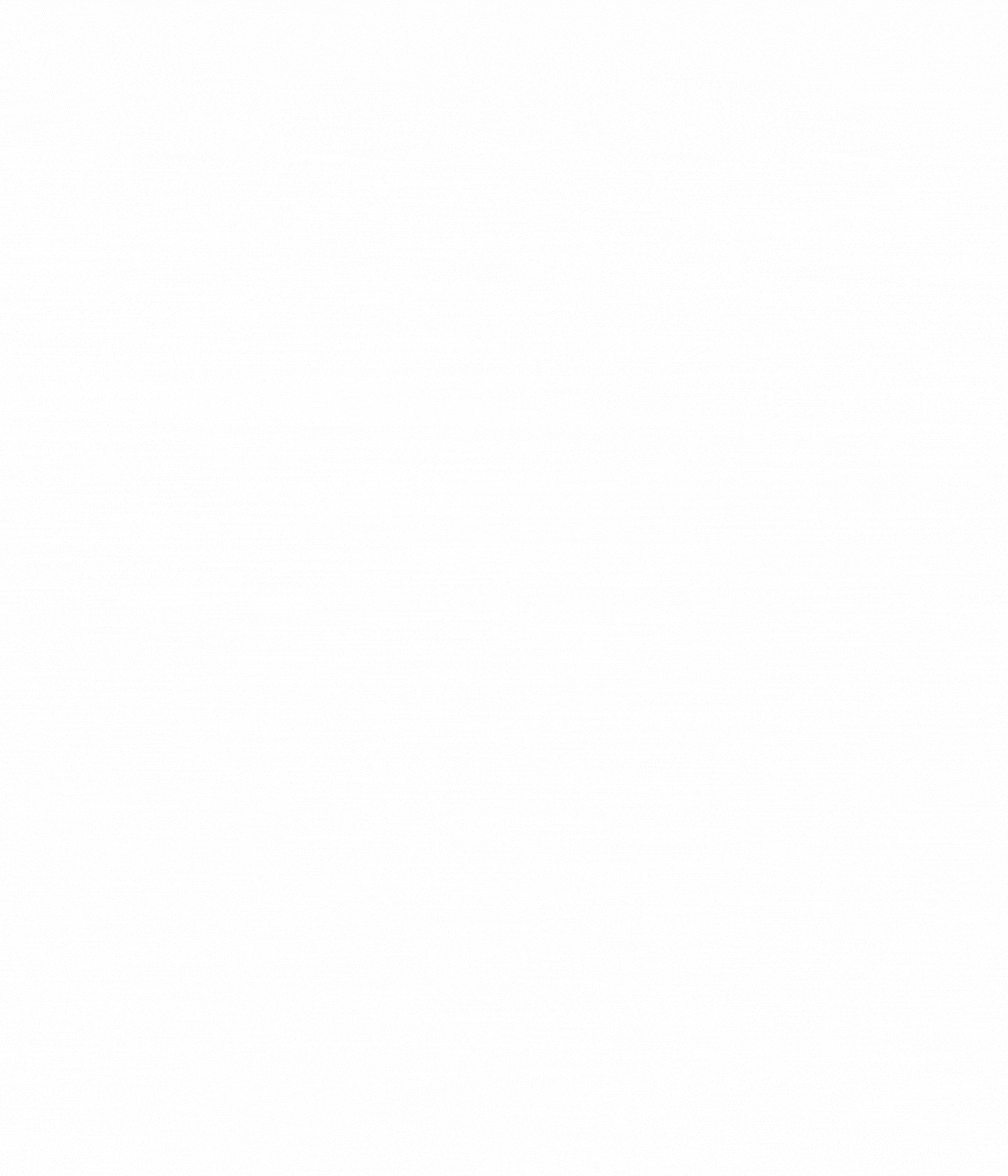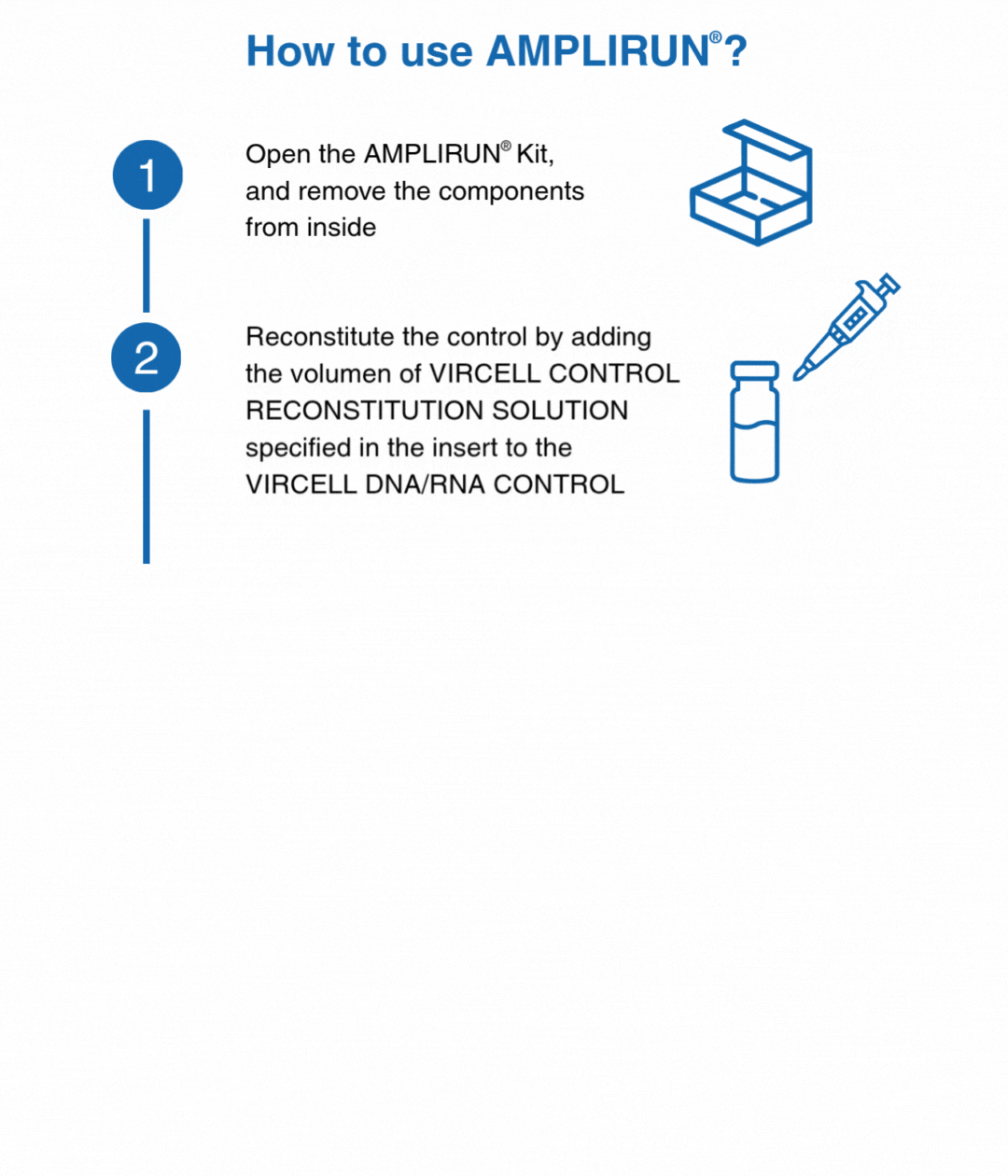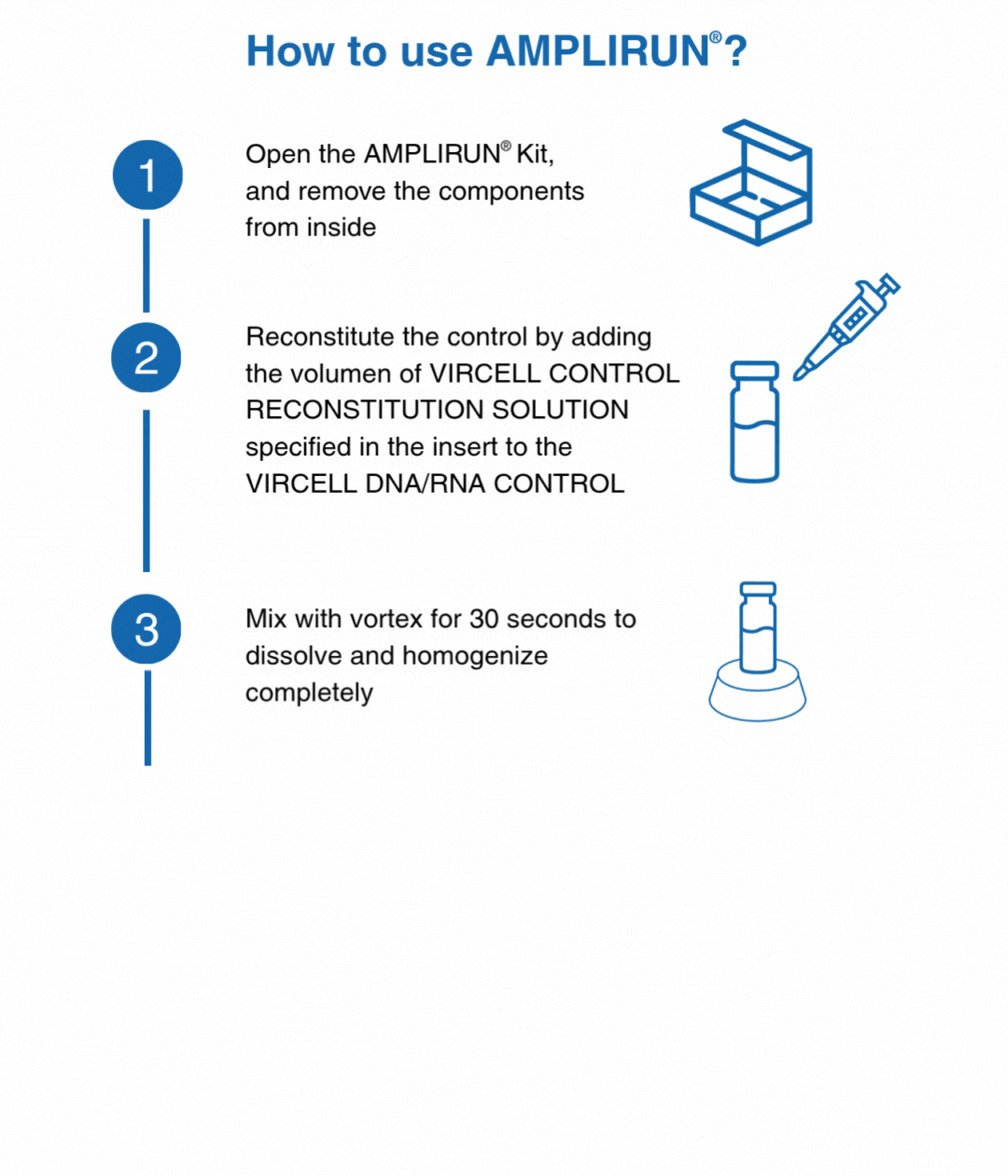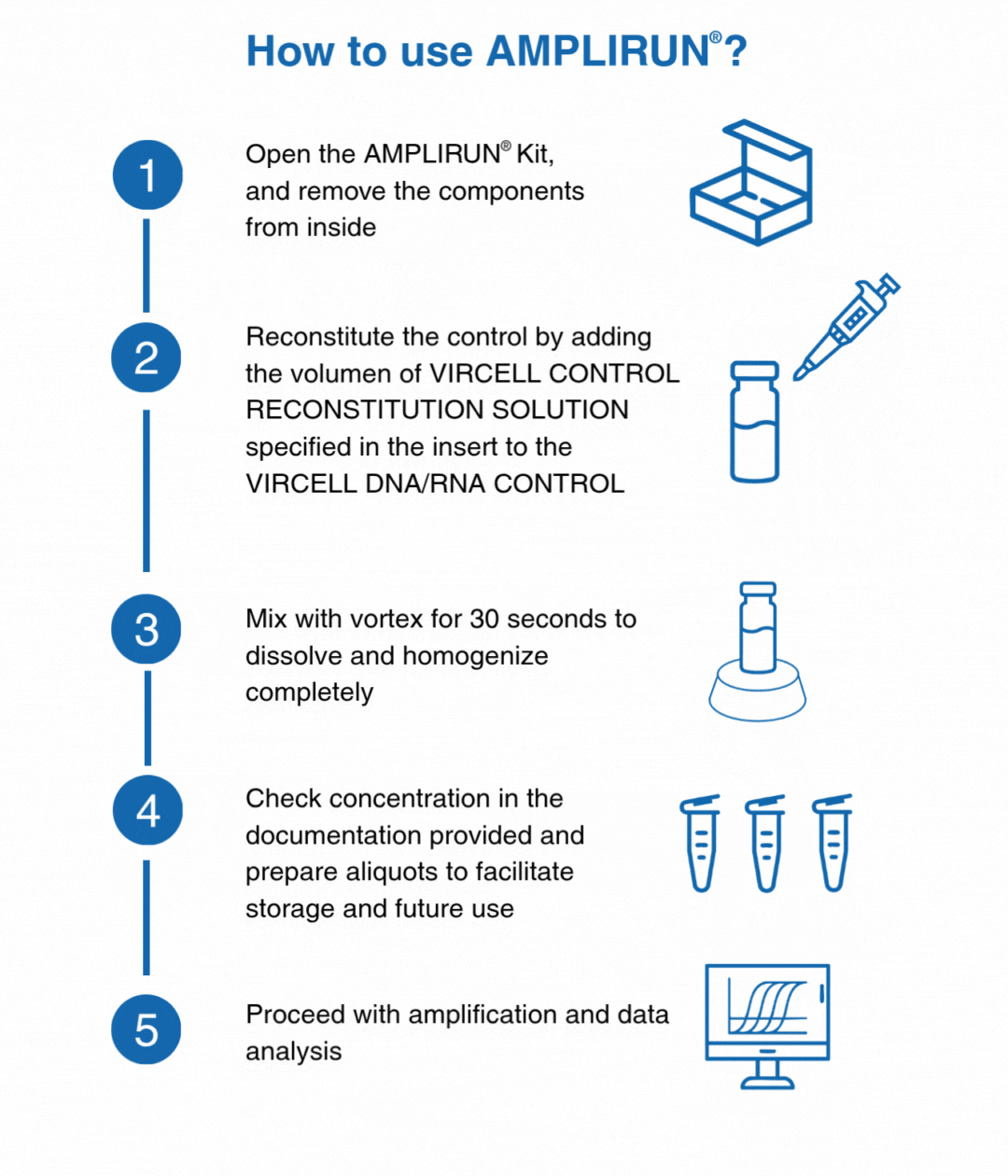
How to use our controls